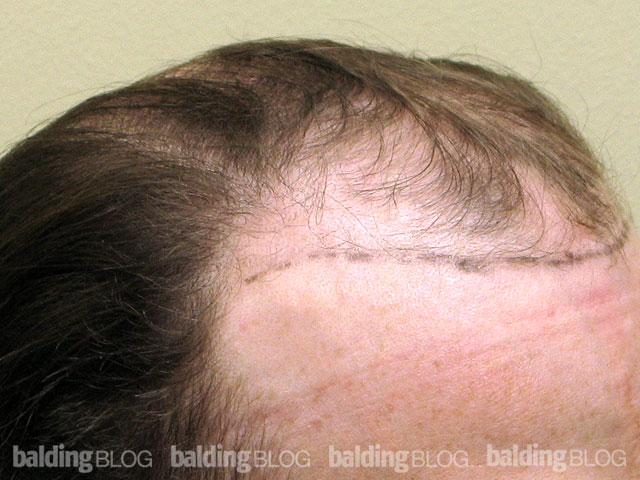
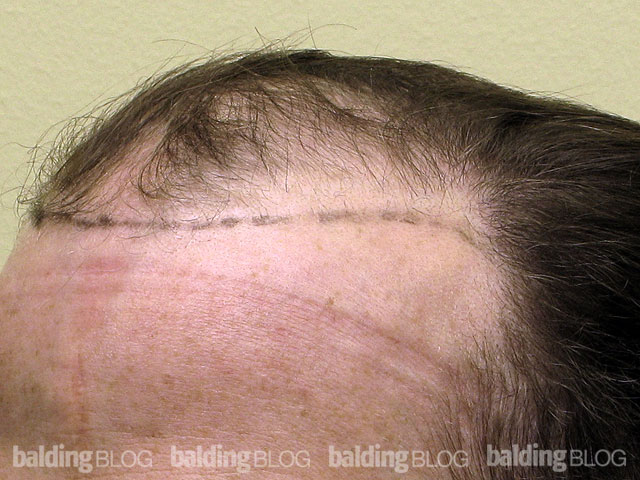
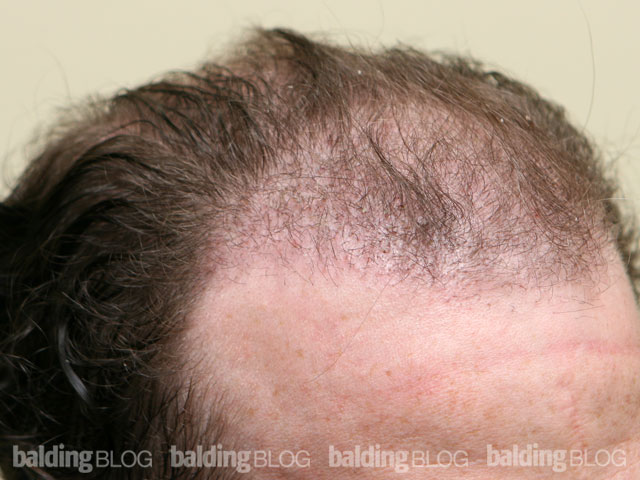
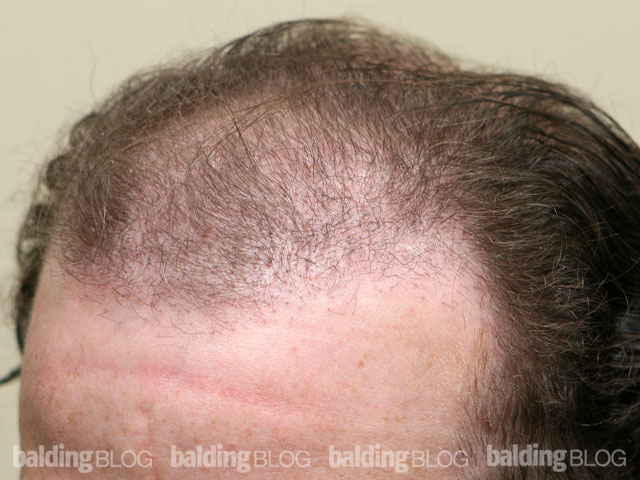
Dr. Rassman,
On Tuesday of this week, Intercytex posted a press release on their website. This press release outlines the company’s clinical results for 2007.
The release does not tell us anything new about the phase 2 trial of ICX-TRC, which is due for completion later this year. It does, however, say the following:
“We believe the continued development of ICX-TRC would best be carried out in partnership with a specialist in the aesthetics field. We do not intend to finance the continuation of clinical and commercial development of ICX-TRC beyond the current Phase II trial and shall seek to sign a partner when we have the complete data package from this trial. Intercytex has granted Bosley, the largest chain of hair transplant clinics in the US, an option to negotiate distribution rights to the product.”
My initial reaction is that Intercytex no longer thinks it is worth developing the treatment any further. What are your thoughts on this?

I do not know enough about Intercytex and its research and development to adequately comment of the matter from a medical perspective. This sounds like a business issue. Drugs or techniques developed by one company often get sold off to another company (common in pharmaceutical industry).

 If you are a woman losing hair, you can use Rogaine Foam (even though it says “for men”). In fact, I generally inform my female patients that they can use the “for men” brand. The only difference I have found is the “blue” vs “pink” color in the packaging and the concentration of the minoxidil. The “for men” stuff is usually 5% and the “for women” is 2%, though women can use the 5%.
If you are a woman losing hair, you can use Rogaine Foam (even though it says “for men”). In fact, I generally inform my female patients that they can use the “for men” brand. The only difference I have found is the “blue” vs “pink” color in the packaging and the concentration of the minoxidil. The “for men” stuff is usually 5% and the “for women” is 2%, though women can use the 5%.